Tremor
Tremor is a neurological movement disorders characterized by involuntary shaking of one or more body parts, often in rhythmic and oscillatory pattern. Tremor can be a disease entity of its own or may be part of a movement disorder such as Parkinson’s disease. In the Systems Neurology group, we focus on the underlying mechanisms of tremor from a pathophysiological point of view. To this end, we utilize electrophysiological, neuroimaging, and brain stimulation techniques as well as clinical data from patients. Our ultimate goal is to improve the treatment of this disabling symptom.
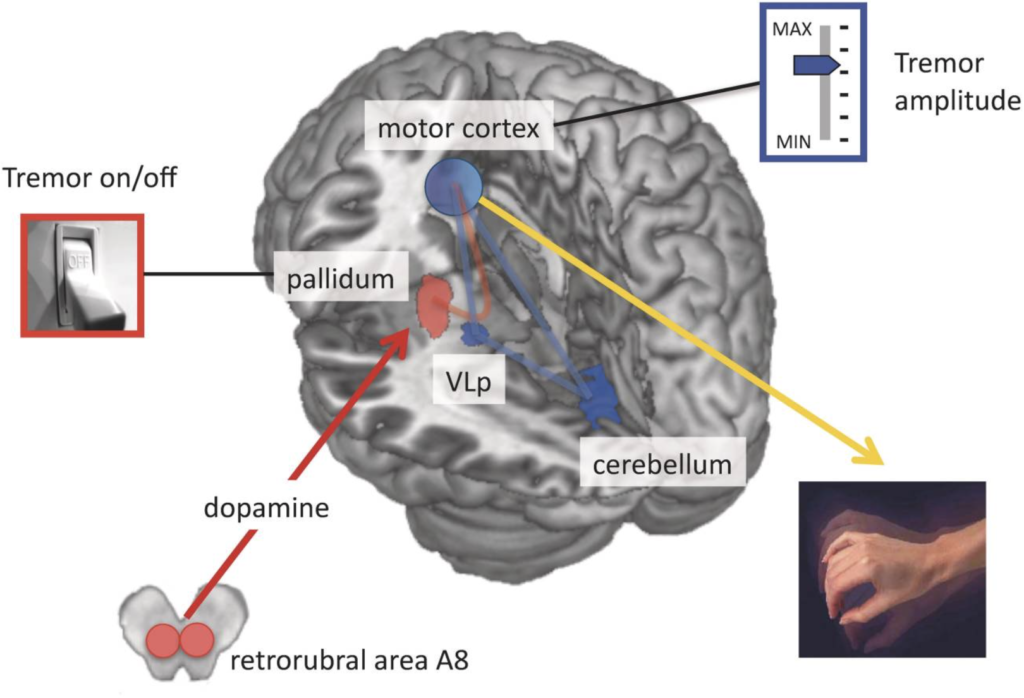
Gaining Insights in Parkinsonian Tremor Pathophysiology
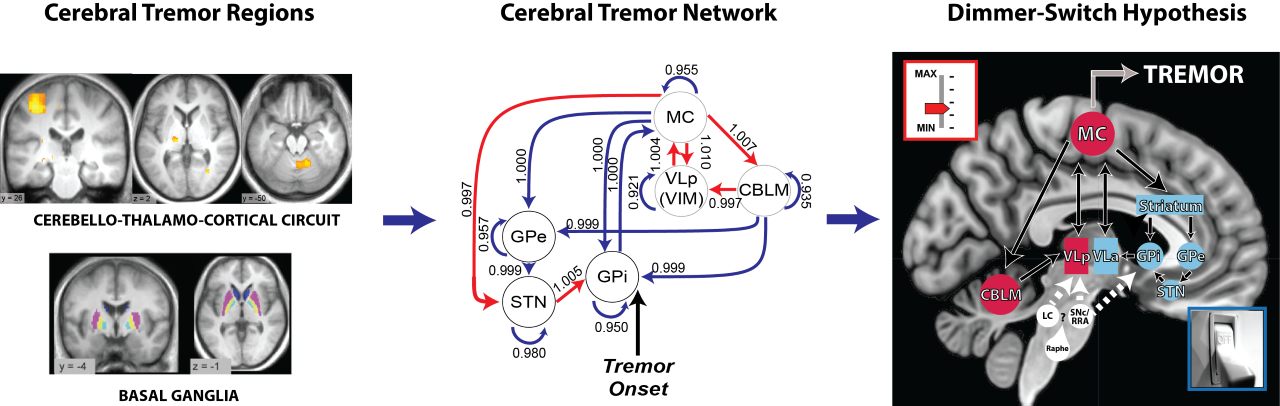
Our aim is to understand the pathophysiology of tremor in Parkinson’s disease from a system-level perspective. That is, which cerebral regions are involved in tremor and how do these regions interact? To this end, we employ various measures including fMRI, electrophysiology, and computational modelling. Using these methods, we find that spontaneous initiations of tremor episodes co-occur with increased basal ganglia activity and that ongoing fluctuations in tremor amplitude cofluctuates with activity in the cerebello-thalamo-cortical (CTC) circuit. This has formed the basis for the dimmer-switch model, where the basal ganglia acts as the ‘light switch’ that turns on the tremor and the CTC-circuit acts as a ‘light dimmer’ which modulates its amplitude.
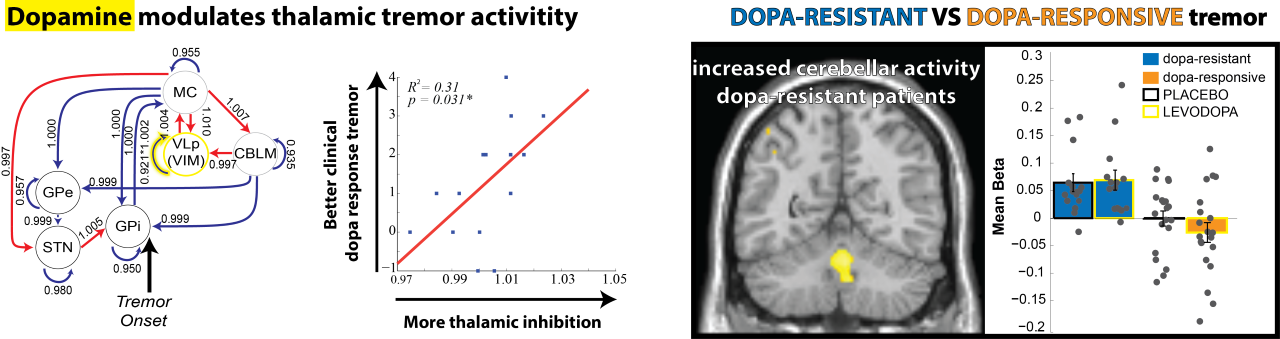
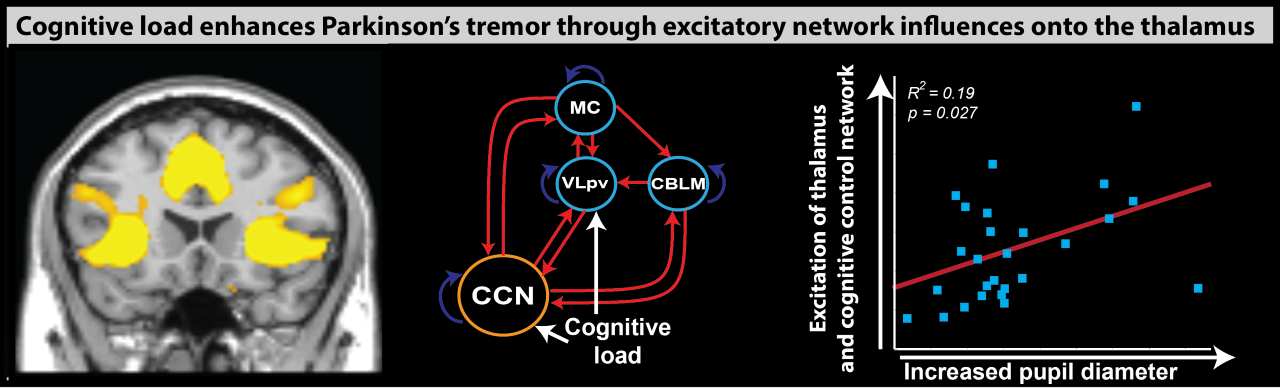
This cerebral tremor network yielded a starting point as to understand how dopaminergic medication is able to reduce tremor-related activity, why the response to dopaminergic medication differs between patients, and how stress amplifies tremor-related activity. We find that dopamine directly acts on the thalamus and that dopamine-resistant tremor is related to increased cerebellar activity, which may unfavorably increase thalamic tremor-related activity. Focusing on the observation that tremor is amplified during cognitive stress, we found that the arousal system increases tremor through direct stimulation of thalamic activity and indirectly by recruiting a cognitive control network.
In future studies, we will not only aim to expand our knowledge of pathophysiological mechanisms, but also aim to bridge the gap between basic and clinical sciences. For example, how can we use pathophysiological parameters to predict clinical phenotypes from a diagnostic or treatment-oriented perspective?
Tremor Progression and Cerebellar Mechanisms
Much remains unknown of the cerebral mechanisms underlying tremor progression. Large multi-centre international studies have shown cross-sectional changes in cortical, thalamic, and cerebellar areas, which may overlap with tremor-related regions and could therefore interact with the central tremor oscillator. Such changes may explain longitudinal increases or decreases in tremor severity. Currently, we are investigating longitudinal changes in clinical tremor severity and its subtypes: resting, postural, and kinetic tremor. Our aim is to explain if and how structural changes in the CTC-circuit explain large inter-individual changes in clinical tremor progression within a well-defined longitudinal Parkinson’s disease cohort. To this end, we will use data from the Personalized Parkinson Project, or in Dutch: Parkinson Op Maat.
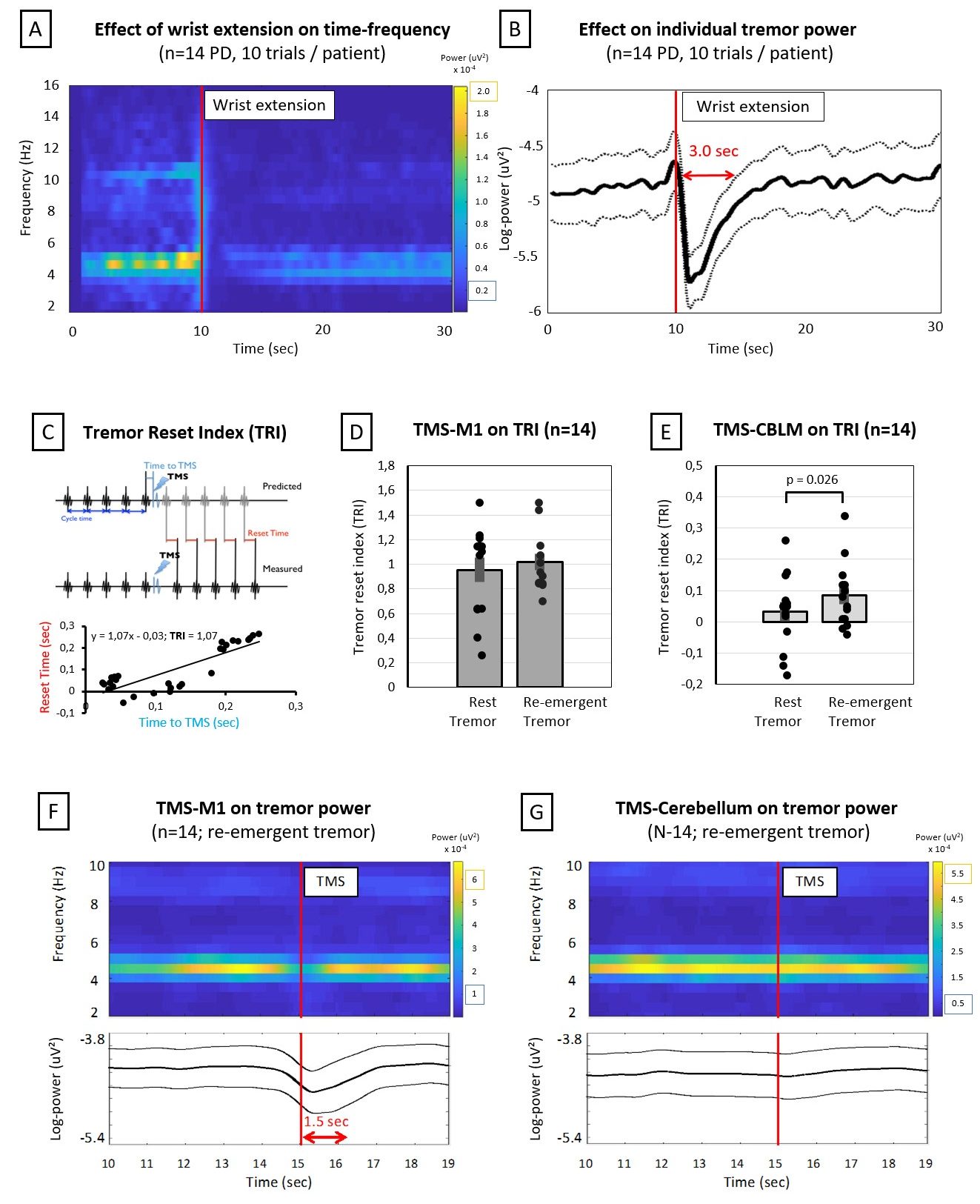
Secondly, we are interested in the cerebellar mechanisms underlying Parkinsonian tremor. Specifically, we are interested in the differential cerebellar contributions in resting and re-emergent tremor. Re-emergent tremor is a common subtype of postural tremor in Parkinson’s disease, which occurs ~2 seconds after stable posturing of the limbs against gravity. This subtype is more debilitating than resting tremor as it directly interferes with daily activities and often does not react well to dopaminergic medication. Using transcranial magnetic stimulation, we showed that the cerebellum was able to reset tremor-rhythm significantly more in re-emergent tremor than resting tremor. In future studies, we will localize the cerebral re-emergent network using combined accelerometry and fMRI. In addition, we will use novel dual-site transcranial alternating current stimulation with the aim to desynchronize and reduce ongoing re-emergent tremor.
Link to published articles:
BAT study
Tremor occurs in up to 55% of dystonia patients. This combination is known as dystonic tremor syndrome (DTS). Treatment for DTS is often unsatisfactory: drugs have low efficacies and side effects and brain surgery is invasive. Botulinum toxin (BoNT) injections are a promising therapeutic option for tremors, but their efficacy varies largely between patients. This highlights the need for personalised treatment in DTS. In our multicentre BAT study, we will follow 72 DTS patients who start 12-weekly BoNT treatment for 40 weeks. Our primary aim is to predict the treatment success of BoNT based on patients’ clinical diagnosis and clinical, electrophysiological and ultrasonographic characteristics. Secondly, we will study the differences between the two clinical manifestations of DTS: dystonic tremor and tremor associated with dystonia. This will give more insight into the underlying pathophysiologies and thereby potential applicability of therapeutic options. Thirdly, we will also explore the validity of ultrasound for muscle selection before BoNT injections, as muscle ultrasound captures muscle movements and discriminates between anatomically overlapping muscles. To summarise our BAT study: we strive towards a more personalised treatment for DTS patients.

Passive monitoring of tremor in Parkinson’s Disease
 Adequate assessment of tremor severity and its context-dependency is necessary for elucidating the individual tremor pathophysiology, and for quantifying treatment effect in clinical trials. Currently, tremor severity is estimated based on physical examinations during routine clinical visits. However, these assessments are subjective and fail to capture the daily fluctuations and context-dependency of tremor. These limitations could be overcome by using wearable sensors that objectively measure tremor severity in daily life. We developed an open-source algorithm that can reliably quantify real-life PD tremor.
Adequate assessment of tremor severity and its context-dependency is necessary for elucidating the individual tremor pathophysiology, and for quantifying treatment effect in clinical trials. Currently, tremor severity is estimated based on physical examinations during routine clinical visits. However, these assessments are subjective and fail to capture the daily fluctuations and context-dependency of tremor. These limitations could be overcome by using wearable sensors that objectively measure tremor severity in daily life. We developed an open-source algorithm that can reliably quantify real-life PD tremor.
We subsequently apply this algorithm to data collected in the Personalized Parkinson Project (or in Dutch: Parkinson Op Maat studie), in which around 500 people were continuously monitored with the Verily Study Watch over two years. We will assess the sensitivity to change of daily-life tremor measures, and compare this to the sensitivity of clinical tremor scores. Furthermore, we will assess the relation between tremor and heart rate measured with the watch, to explore the stress-sensitivity of tremor in daily-life. Ultimately, this may aid in the development of more personalized treatment for tremor in Parkinson’s disease.
The effect of the STIL Orthosis on action tremor severity in Parkinson’s disease
While PD tremor usually occurs at rest, 46-93% of patients also have action tremor, occurring during voluntary movements. By interfering with daily activities, action tremor can reduce independence and quality of life. Action tremor often does not respond to
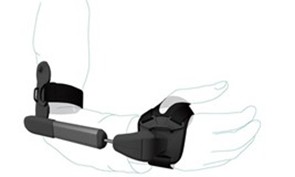
dopaminergic medication, and stereotactic surgery is invasive and unsuitable for many patients. The STIL orthosis, a CE-certifed device, aims to reduce tremor by dampening wrist flexion-extension and forearm pronation-supination. It features dampeners that limit high-frequency movements, such as tremor, while maintaining voluntary movements. In patients with essential tremor (n=24), the STIL orthosis significantly reduced action tremor severity during daily activities (Mugge et al., 2025). This suggests that it could be a promising treatment for PD action tremors as well. In the STIL-PD study, we will assess 25 PD patients with action tremor to evaluate the effectiveness of the STIL orthosis. Our primary aim is to investigate differences in tremor severity during activities of daily living while wearing the orthosis, compared to baseline (no device). Secondly, tremor characteristics are measured with accelerometry and EMG. If proven effective, the orthosis could be an important solution for the large group of PD patients who have a disabling action tremor, by reducing the impact of their tremor on activities of daily life.
Link to trial registration: https://clinicaltrials.gov/study/NCT06886178?term=orthosis%20parkinson&rank=1
