Compensation and disease progression
Neurodegenerative disorders such as Parkinson’s disease typically progress over very long periods of time. As these disorders progress, brain network dysfunction becomes more and more pervasive, which drives the worsening of symptoms. However, given its neuroplastic properties, the disordered brain is able to adapt and alter the functioning of its networks in ways that counteract, or compensate for, pathological dysfunction. The eventual decline of these compensatory mechanisms may be key to understanding symptom progression in neurodegenerative disorders.
Cerebral Dysfunction and Compensation
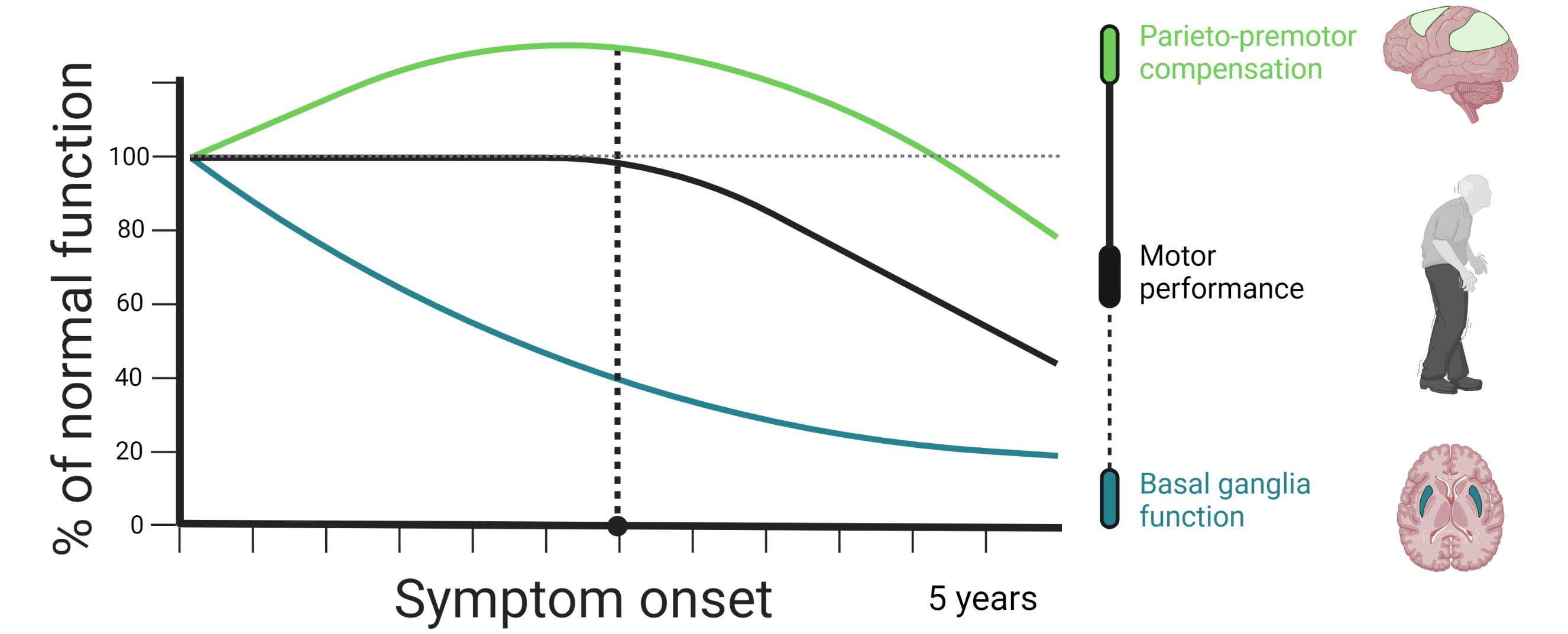
Basal ganglia dysfunction is often thought of as the main pathological mechanism that gives rise to motor symptoms in Parkinson’s disease. According to this view, motor symptoms emerge when the cortico-striatal circuit that initiates and executes movements is disrupted due to dopamine depletion in motor-territories of the striatum (i.e. the post-commisural putamen). At the moment, this particular model has some notable shortcomings that limit its ability to explain why motor symptoms emerge and why their progression rates differ so much between individual patients. First, substantial dopamine depletion takes place many years prior to the onset of motor symptoms. Second, several longitudinal studies have failed to find an association between striatal dopamine depletion and motor progression. Third, Parkinson’s disease pathology affects multiple neurotransmitter systems, not just dopaminergic ones.
Functional imaging studies in Parkinson’s disease have shown that patients tend to rely more heavily on parieto-premotor regions during motor tasks in comparison to healthy controls. This has led to the idea that individuals with Parkinson’s disease may be able to preserve motor control for a limited time by using parieto-premotor resources to compensate for basal ganglia dysfunction. According to this view, the emergence of motor symptoms in Parkinson’s disease may be tightly linked with the integrity of parieto-premotor compensation.
We are currently employing a novel action selection task in combination with longitudinally acquired functional magnetic resonance imaging data, acquired at baseline and at two-year follow-up, to investigate the role of basal ganglia dysfunction and parieto-premotor compensation in Parkinson’s disease. Our first aim is to demonstrate Parkinson’s disease-specific changes in brain function. We will achieve this by comparing longitudinal change in motor-related activity between patients and healthy controls. Our second aim is to investigate whether individual differences in motor symptom progression is associated with longitudinal changes in basal ganglia dysfunction, parieto-premotor function, or both. Here, we will directly compare the strength of the association that change in basal ganglia and parietal cortex activity has with change in motor symptoms.
JPND funded CONTROL-PD
Though most known for its motor symptoms, it is becoming increasingly clear that Parkinson’s disease is a diverse disorder affecting a wide range of bodily and cognitive functions. These symptoms can arise at different stages of the disease and progress at different rates, leading to the hypothesis that Parkinson’s disease consists of various subtypes. The National Institute of Health has established subtyping and progression as one of the top priorities in Parkinson’s disease research. The CONTROL-PD consortium project was awarded 1.5M€ from the EU Joint Program for Neurodegenerative Disease to test novel hypotheses regarding subtyping and progression rates in a large multi-center dataset. Apart from the Netherlands, CONTROL-PD consists of collaborators from research institutes in France, Canada, Israel, Australia, Germany, and Portugal. Together, the cohorts from the different sites include over 3600 individuals with Parkinson’s disease with multi-modal data including clinical, behavioral, and neuroimaging measurements. To tie these cohorts together, an online follow up of the patients will be conducted which will utilize a novel cognitive task battery and questionnaires which will allow the investigation of a new subtyping dimension. CONTROL-PD has partnered with RobustCircuit to develop and execute this large scale online data collection.
Genetic Influence on Cerebral Dysfunction and Compensation in PD
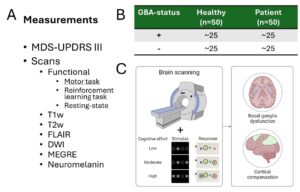
Glucocerebrosidase (GBA) mutations are the most significant genetic risk factor for Parkinson’s disease (PD), often leading to a more aggressive progression of the disease. Our research project aims to uncover why PD associated with GBA mutations tends to follow a more severe clinical course. We focus on a unique cohort from the Luxembourg Parkinson Study, which includes both symptomatic and asymptomatic individuals with and without GBA mutations, offering a rare opportunity to investigate early, subclinical changes of basal ganglia function.
Using advanced neuroimaging techniques such as functional MRI (fMRI) and several structural measures (Neuromelanin, DWI, MEGRE) we investigate the neuronal mechanisms underlying motor and non-motor symptoms in PD. Specifically, we explore how these symptoms manifest in patients with GBA mutations, and how the brain’s compensatory mechanisms differ between those with and without these mutations. By comparing brain activity during specific cognitive and motor tasks, as well as the effects of dopaminergic medication, we aim to identify early biomarkers for disease progression. We then intend to validate the knowledge gained from this on a larger number of patients in the cohort of the Personalized Parkinson’s Project.
This project is part of a broader international collaboration of the DCCN with partners of the NCER-PD program. Our ultimate goal is to enhance understanding of the genetic and phenotypic variations in PD, leading to improved diagnosis and treatment strategies for patients.
