Stress
Stress is a normal physiological reaction, needed to anticipate threats. However, chronic stress can lead to psychiatric disorders like depression and anxiety. In people with Parkinson’s disease (PD), the prevalence of such disorders is exceptionally high and symptoms worsen in stressful situations. Also, animal models of PD suggest that chronic stress accelerates disease progression. In our research, we use fMRI to investigate the cerebral mechanisms underlying this increased stress-sensitivity in PD. Also, we explore how reducing stress (pharmacologically and non-pharmacologically) affects PD symptom progression.
The effect of stress and stress-reducing strategies on PD
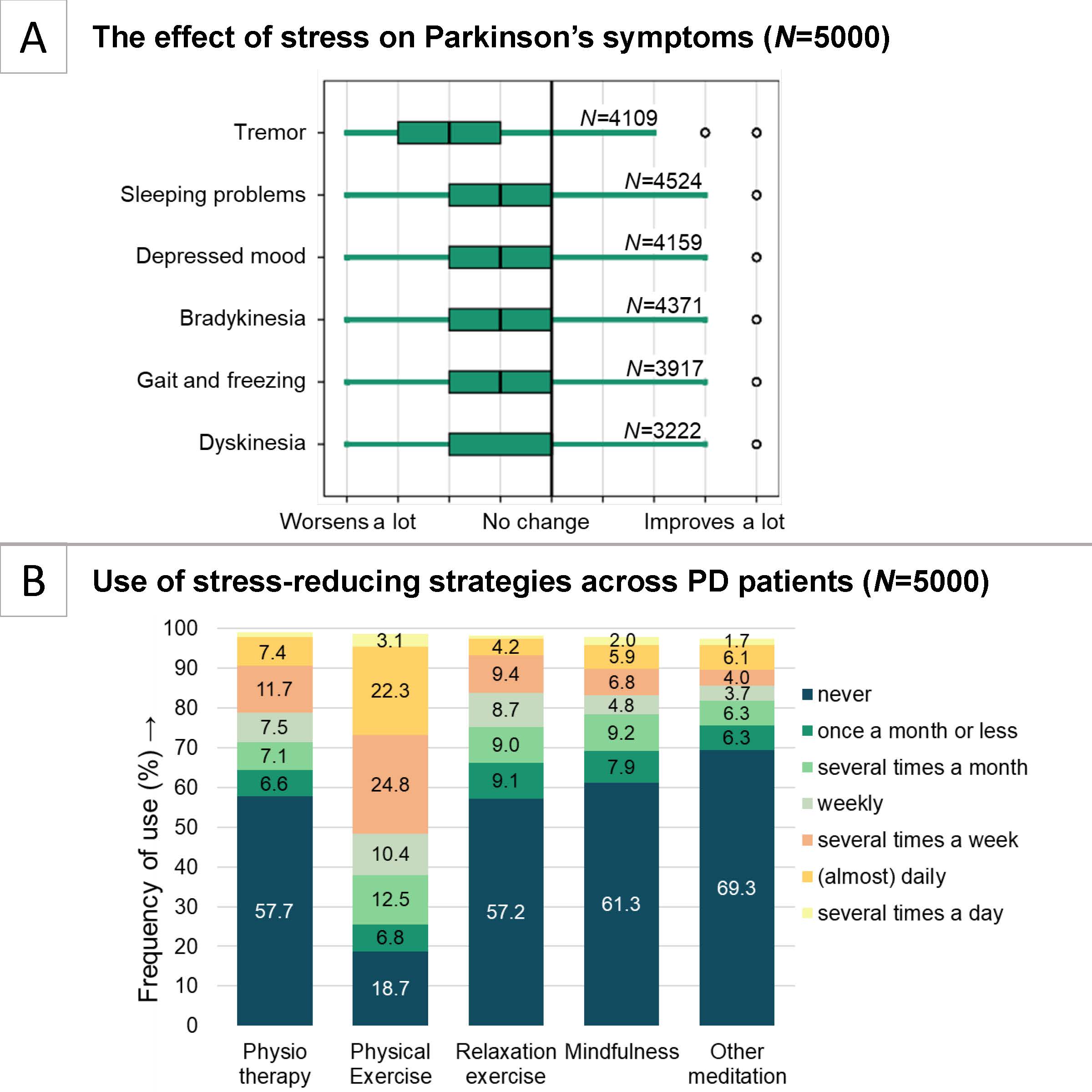
Many people with PD notice that symptoms worsen during stress, and experience stress-related neuropsychiatric symptoms. In 2019-2020, we performed a large online survey study in 5,000 PD and 1,292 healthy controls participating in the FoxInsight cohort (US). In addition, the same questionnaire was filled out by 326 PD and 52 healthy controls through ParkinsonNEXT (NL). With this survey, we investigated which personal and disease characteristics are associated with perceived stress in PD, which PD symptoms are sensitive to stress, and we assessed benefits of stress-reducing strategies. People with PD perceived more stress than controls. Among patients, stress was correlated with increased rumination (R=0.65), lower quality of life (R=-0.56), lower self-compassion (R=-0.65), and lower dispositional mindfulness (R=-0.48). Stress significantly worsened both motor symptoms and non-motor symptoms, with the largest effect on tremor. Physical exercise was most frequently used to reduce stress (83.1%). Mindfulness was practiced by 38.7% of PD respondents in the US and 20% in NL, who noticedb improvement in both motor and non-motor symptoms, with the strongest effects on anxiety and depressed mood. These findings justify further controlled studies to establish the merits of mindfulness and other stress-alleviating interventions.
Link to published article: Van der Heide, A., Speckens, A. E., Meinders, M. J., Rosenthal, L. S., Bloem, B. R., & Helmich, R. C. (2021). Stress and mindfulness in Parkinson’s disease–a survey in 5000 patients. NPJ Parkinsons Dis, 7(1), 1-10.
The effect of mindfulness on PD
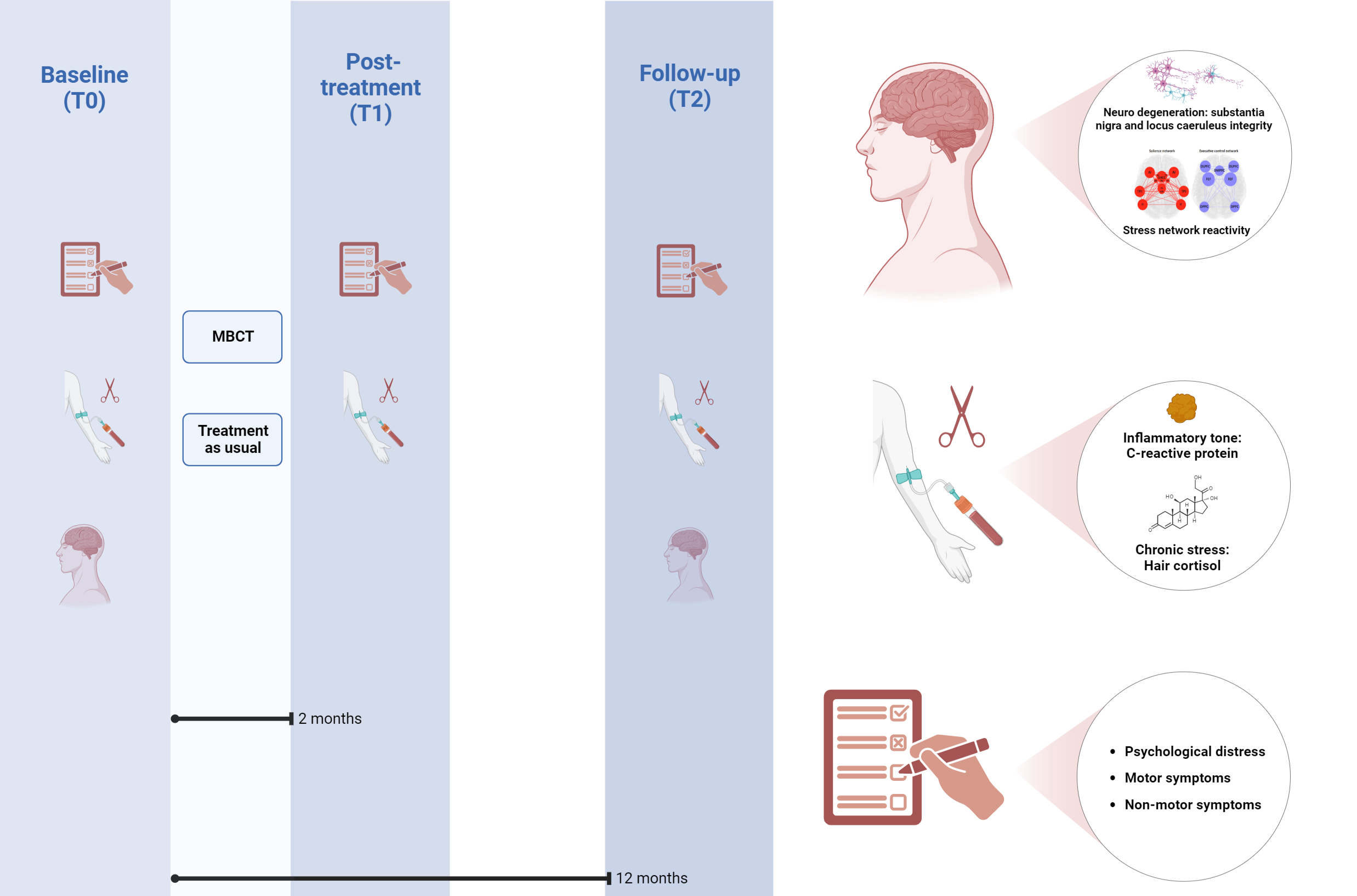
In the MIND-PD study, we perform a large randomized controlled trial to systematically test the effects of Mindfulness-Based Cognitive Therapy (MBCT) on the clinical (symptomatic) and neurodegenerative course of PD. Importantly, we will not only investigate whether MBCT is effective in reducing stress, but for the first time explore the cerebral mechanisms underlying the effects of stress (reduction) on PD and PD progression. In a group of 124 patients, we compare the effects of MBCT to treatment as usual with regards to stress-related symptoms (measured by the Hamilton Anxiety and Depression Scale), disease severity, cerebral markers of neurodegeneration, systemic inflammatory tone and hair cortisol (a marker for chronic stress). Each participant is followed for 1 year, including three extensive measurement time-points (at baseline, after 2 months and 12 months after baseline). MBCT is provided by trained mindfulness trainers at the Radboudumc Centre for Mindfulness and consists of 8 weekly training sessions, daily homework, and regular booster sessions. If proven to be effective, MBCT can be applied as a new and cost-effective therapy for people with PD. Insight to the cerebral mechanisms of mindfulness based interventions and stress reduction in PD can pave the way for developing new, mechanism-based interventions and will help to uncover the nature of the effects of stress on PD. Link to trial registration.
Heterogeneity in medication effects on cognition in PD
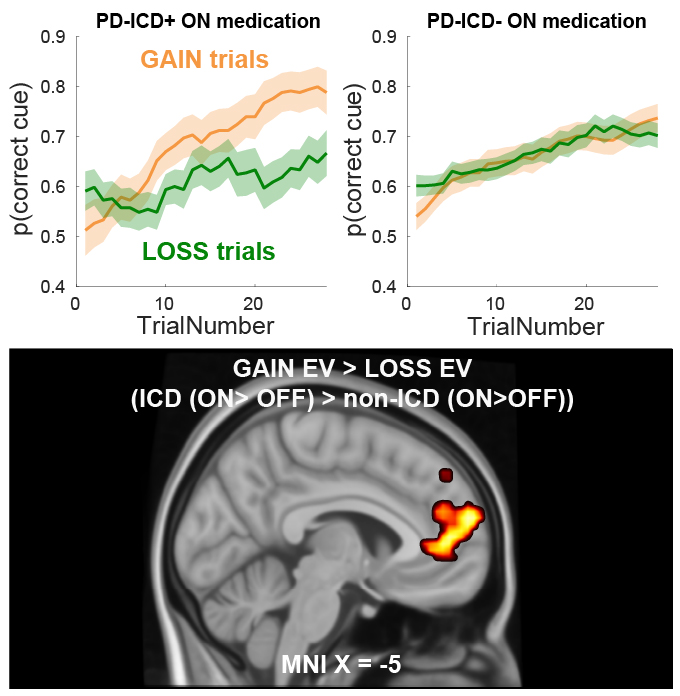
Foremost, people with PD are treated with levodopa and dopamine receptor agonists, which are able to alleviate both motor and cognitive symptoms in PD. Simultaneously, they contribute to both cognitive improvements and deficits. For example, patients ON Medication show increased errors on associative learning tasks and reversal learning tasks. Most likely, through medicinal overstimulation of relatively intact dopaminergic reward pathways. This leads to an oversensitivity for rewards, compared to punishments. In severe cases patients develop impulse control disorders; manifesting as pathological gambling, eating, shopping and/or hypersexuality. In terms of quality of life and stress, impulse control disorders (ICDs) are among the most impactful symptoms. Approximately 14% of patients develop ICDs, often after increasing the use of dopamine receptor agonists. In fact, PD patients with ICDs are particularly susceptible to medication effects leading to reward oversensitivity. To build towards a predictive model of ICD susceptibility, we investigate the role of medication, reward learning, inflammation and neural reward signaling in people with PD.
Link to published article: Sayalı, & Cools, R. (2023). Impulse control disorder in Parkinson’s disease is associated with abnormal frontal value signalling. Brain, awad162, 1-14.
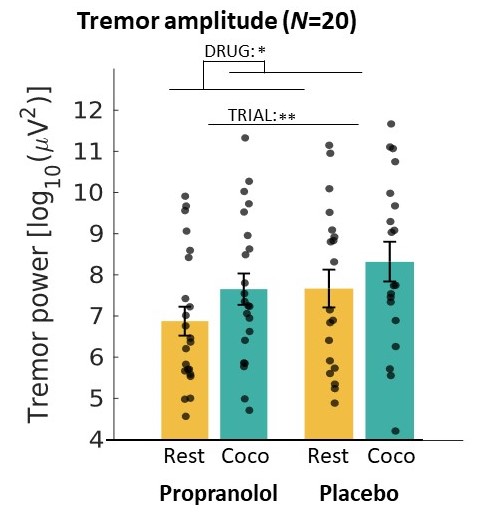
The role of the noradrenergic stress system in PD tremor
The PD resting tremor often does not respond well to dopaminergic treatment. Better pathophysiological solutions of the PD tremor are needed as a basis for improved treatment strategies. We have several indications that the noradrenergic stress system plays an important role in the PD resting tremor. A first indication is that stress clearly worsens tremor, and that the severity of tremor does not relate well to the amount of dopamine deficiency. In addition, many patients experience that their usual dopaminergic medication is less effective in stressful conditions. Clinically, this shows an important problem: patients suffer most from their tremor during psychological stress, but this is when available treatment is least effective. The resting tremor has been shown to be initiated in the basal ganglia, and that the cerebello-thalamo-cortical circuit regulates the amplitude of the tremor. We expect that the noradrenergic system amplifies tremor activity in tremor network in the brain. We used propranolol, a beta-blocker that suppresses noradrenergic neurotransmission, as a measure of noradrenergic activity to further study this, and to see where in this model noradrenalin acts.
Participants lay in MRI scanner while doing a cognitively stressful task, where we activated the noradrenergic system by letting patients make mental calculations. We measured cerebral activity, tremor amplitude, pupil diameter and heart rate. With this study, we are gaining more insight into the relationship between stress and tremor. This could lead to new treatments for tremor in the future, where treatment could specifically focus on the noradrenergic system thereby lowering the effect of stress on tremor.
